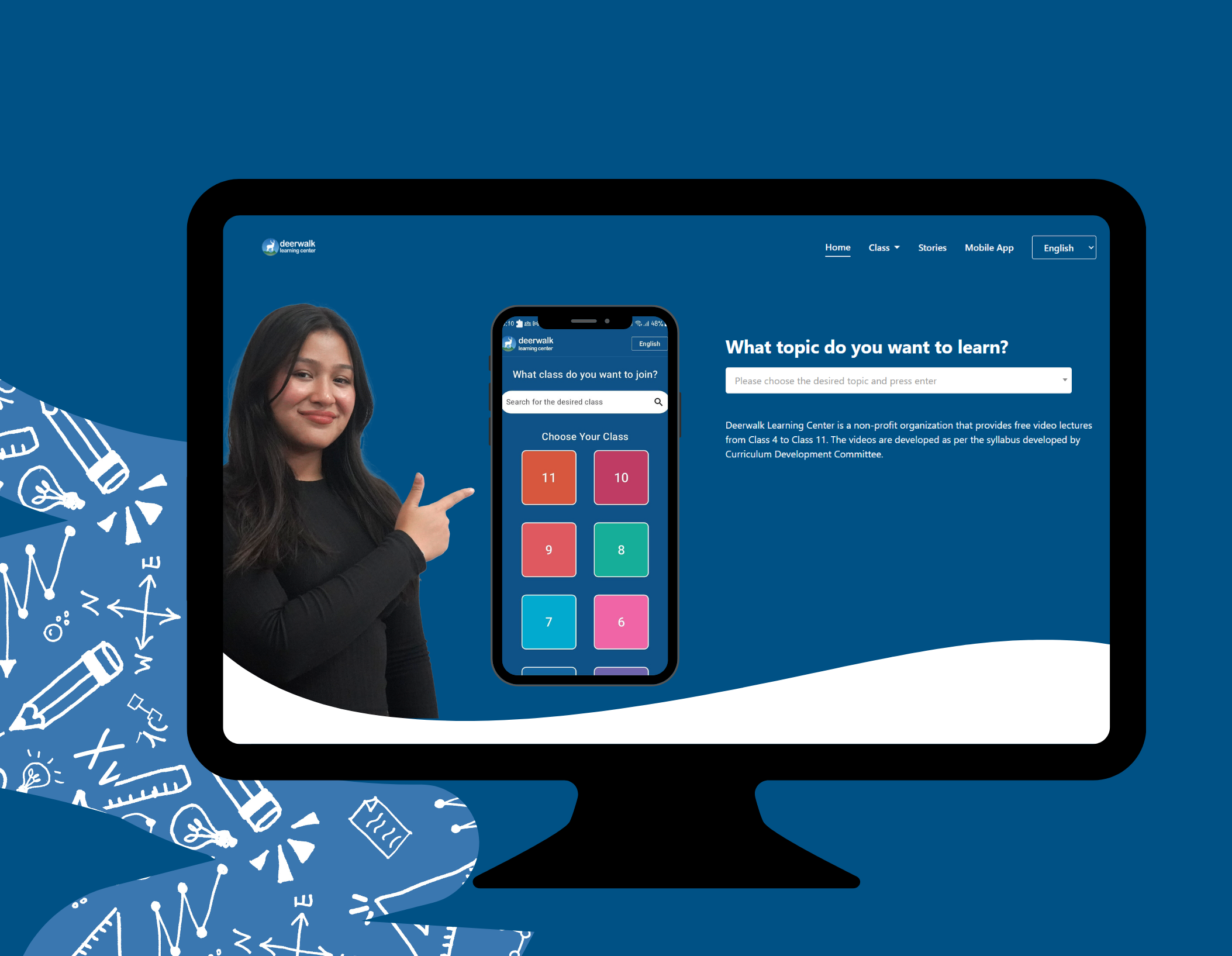
What topic do you want to learn?
Deerwalk Learning Center is a non-profit organization that provides free video lectures from Class 4 to Class 11. The videos are developed as per the syllabus developed by Curriculum Development Committee.
Testimonial
Content Distribution Partner